124Uploads
33k+Views
8k+Downloads
Chemistry

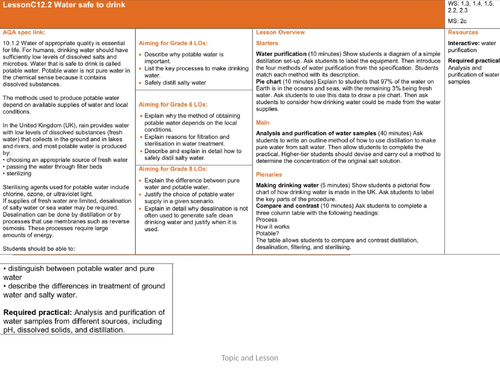
C12.1 Making water safe to drink
Starter: differentiated starter questions with answers
Main: Discussion which water from the pictures would you rather drink.
Define: potable water
Carry out required practical
answer questions
green pen, self assess questions
discuss why water is so important.
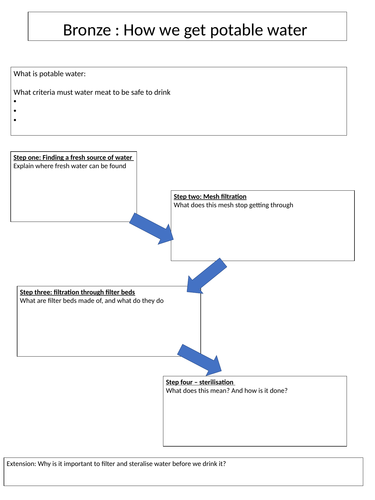
Complete worksheet to show how potable water is made (differentiated worksheets)

Potable water differentiated worksheet
Bronze/silver/gold differentiated worksheets
To show how potable water is made
Aimed at low ability students
Bundle

C11 - The earths atmosphere
All resources aimed at foundation students
C11.1 + C11.2 combined into one lesson
C11.3 greenhouse gases
C11.4 global climate change
C11.5 atmospheric pollutants

C11.5 atmospheric pollutants
aimed at foundation level 4 targets
Differentiated bronze/silver/gold starter
Main: recap complete and incomplete combustion, put piece of pot over blue/yellow flame on bunsen burner
Students fill in worksheet, to say what they saw and the difference between incomplete and complete combustion
Students fill in information grid using textbooks, to find the causes of the atmospheric pollutants and the problems with them
Answers for students to check their answers with

C11.4 global climate change
Aimed at foundation students targets of level 4
Starter: differentiated bronze/silver/gold starter based on previous lessons in the topic, answers on following slide
Main:
Watch video, write a list of as many problems form global warming
think, pair, share: what is a a carbon footprint - write defintion
discuss what the different countries of the world are trying to do to combat global warming
Students fill in an information sheet from what they have learnt, differentiated extension questions with answers if students complete
Plenary: higher or lower, interactive, do you think the following country has a higher or lower level of renewable energy use

C11.3 greenhouse gases
Aimed at foundation students, targets of level 4
Starter: differentiated bronze/silver/gold starter questions with answers
Main:
Draw the greenhouse effect using info from powerpoint. extension question to challenge students
Fact hunt: questions to answer using textbook
Video on deforestation, questions to answer from video
Plenary: animated true/false statements

C11.1 History of our atmosphere
Aimed at foundation students. targets of level 4
Information about how the earths atmosphere was created, then a story board for students to fill in using questions from the version on the board.
Extension questions included to challenge students
extension sheet if students finish.
6 mark question on this topic for students to practice exam technique as a plenary

C3.5 covalent bonding - foundation
aimed at a lower ability class
Starter Activity
Mark homework
Main –
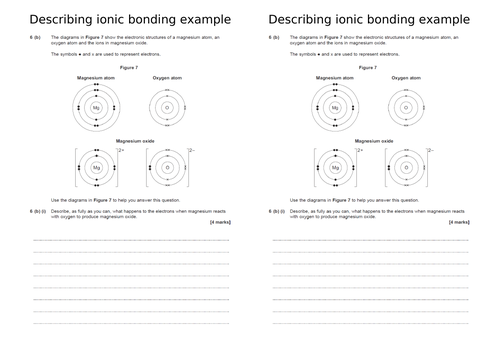
Think pair share – spot the difference between ionic and covalent bonding. Then write down definition of covalent bonding
Watch video, only watch until 2 minutes. Answer questions whilst video is going. Goo through and green pen answers after
Go through the two examples on the board and show what happens
Students then have a go at completing their worksheet to show covalent bonds
Extension: decide if the compounds are ionic or covalent and explain how they know
Plenary
Plenary, fill in the gaps and go over answers

C3.4 Giant ionic structures - foundation
aimed at a lower ability class
Starter Activity
Using previous knowledge make predictions about ionic compounds
Main –
Go through and discuss giant ionic lattices
Students move around the room to find the information sheet to find out if there prediction was correct or not and why
Practical with a dissolved ionic compound to show it can conduct electricity. Make sure say that molten ionic compounds too and what a molten ionic compound is
Plenary
Exam question plenary

C3.3 Ionic bonding - foundation
Aimed at a lower ability class
Starter Activity
Ions recap, what charge do elements have in different groups
Stretch question to think about how group 1 and 2 will interact with group 6 and 7
Main –
Go through the video and answer the questions, go through answers
Practice drawing ionic compounds Extension: explain the difference between KBr and K2O
Go through the answers which are on the slides
Exam question practice to move onto if have a chance
Plenary
4 mark exam question – peer assess
Good to show the detail they need in their answers

C3.2 atoms into ions - Foundation
aimed at a lower ability class
Starter Activity
Identify the atom on the board
Stretch – how do you know which atom it is
Main –
Recap how to find out electron/proton/neutron numbers and how to draw electron configuration. Think pair share, what can the two atoms do to have full outer shells
Students explain in their books what a positive and negative ion is
3 quick questions – green pen mark
Go through rules so we always know the charges of common ions
Worksheet to complete – label periodic table with rules and then practice drawing ions
Extension: describe and explain how ions are formed
Plenary
Exam question plenary – peer assess

C3.1 States of matter - Foundation
Aimed at a foundation class
Starter Activity
Draw what they can remember about the states of matter, go through what the particle pictures should look like
Main –
Complete worksheet on properties of solids, liquids and gases - differentiated versions
Stick in particle diagrams – students add labels and arrows to show what the state changes are. Then use textbooks and what we have discussed to describe what happens to the particles during changes of state.
Explain cooling curves, students fill in the blanks on their cooling curve
Extension for students looking at cooling and heating curves and melting and boiling points
Plenary
Little quiz to finish with

C1. 8 electronic structures
Aimed at a mixed ability year 9 class
Starter Activity
Exam question starter – go through and green pen
Main –
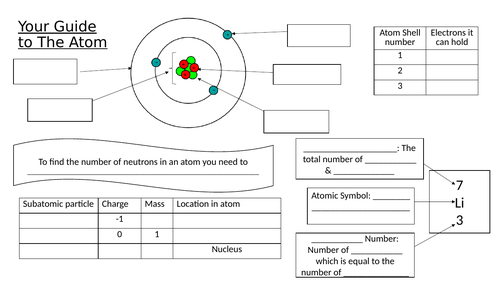
Recap where to find the number of electrons an atom has, go through electronic structure rules. Do a few examples on the board
Two worksheets – first worksheet students need to find out the number of protons, neutrons and electrons each element has, the second they need to draw the electronic structures
Extension: explain why sodium and lithium might react in a similar way
Write a step by step guide for somebody to explain how you draw electronic structures
Plenary
Exam question plenary – spot mistakes in the electronic structures

C1.7 Ions, atoms and isotopes
aimed at mixed ability year 9 class
Starter Activity
Differentiated Bronze/silver/gold questions from previous lesson
Main –
Think, pair, share – how big is an atom. Copy down the size of an atom and nucleus intho their books
Copy key words need to be able to define ion and isoptope for level 4
Explain that atoms can lose or gain electrons. And what happens if they do. Students make notes on this, then go through a few examples.
Students practice working out electron/proton/neutron for ions
Extension task for students who finish
Explain what an isotope is, do one worked example of finding proton/neutron numbers then their go at finding how many protons and neutrons are in carbon isotopes
Exam practice if time
Plenary
Identify whether the pictures are atoms, ions or isotopes

C1.6 structure of the atom
aimed at a mixed ability year 9 class
Starter Activity
Put atom structures in the correct order thinking back to last lesson
Main –
Work through work sheet in steps. To ensure maximum understanding
Chase the element – race task to find out what each element around the room is, identifying proton neutron and electron number and matching it with an element on the periodic table
Make a model of an atom using string, and card (cut card into circles use string to attach them together)
Plenary
Tweet what you’ve learnt tday

C1.1 History of the atom
Aimed at a mixed ability year 9 class
Starter Activity
Draw and label the atom – discuss that its changed over time
Main –
Watch video of history of the atom – try and list the scientists and their discoveries
Use textbooks or ipads to research the history of the atom and fill in the timeline. Differentiated version, blank version for HA students and LA version with question prompts
Extension fill in the gaps sheet
Plenary
Draw the current atom from memory and label it

C1.4 fractional distillation and paper chromatography
aimed at a mixed ability year 9 class
Starter Activity
Match key words to definitions – go through answers
Main –
Students watch demo of fractional distillation. Explain uses/whats happening at each point. Students the write an explaination of how they can obtain pure ethanol
Set up chromatography experiment, whilst waiting for it to develop write a step by step method for the practical. Go over basics, this will be revisited as chromatography is a required practical.
Plenary
Tweet one thing they have learnt today

C1.2 Separating mixtures
Aimed at a mixed ability year 9 class
starter:
Differentiated starter task from previous learning. Bronze = level 4 silver – level 6 gold = level 8 (to be gaining a level 8 answer needs to be really detailed) students have done this at KS3
Go through and green pen answers
Main
Talk about safety for the practical, demo practical with distillation to get pure water
Students carry out rock salt practical to get salt
Answer questions when done, extension question available also
Plenary
5 mins
Identify the three processes we have learnt about today
Homework - differentiated
Summary sheet on separation techniques

C1.2 Chemical equations
Aimed at a mixed ability year 9 class
Worksheets are from TES
Starter Activity
Answer starter questions
Main –
Go through state symbols, make a note of what each means
Explain the conservation of mass – need to write down the defintions
Stretch: why do some reactions appear to gain/lose mass
Go through steps on how to balance equations. Supporting worksheets to build up. Make sure there is an emphasis on what the ‘big’ and ‘little’ numbers mean. Do examples at each step, then get students to complete the rest
Extension sheet of harder equations to balance for students flying through
Plenary
Plenary – make rules for balancing equations