497Uploads
167k+Views
71k+Downloads
Chemistry

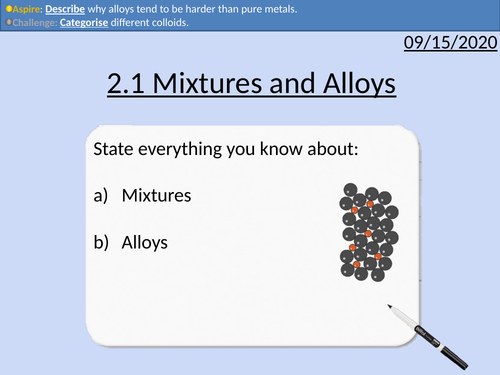
OCR Applied Science: 2.1 Mixtures and Alloys
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.1 of Science Fundementals of the OCR Applied Science Spec.
Types of mixtures to include solutions, colloids and suspensions
Difference between colloids and suspensions in terms of particle size
Uses of common colloids in nature and medicine
Types of colloids to include aerosols, emulsions, foams, gels and sols
Significance of colloids in nature and medicine
Alloys as mixtures of metals
The character and features of alloys
Uses of common alloys to include amalgam, solder, bronze, titanium alloy

GCSE Chemistry: Filtration and Crystallisation
This PowerPoint presentation with worked examples and student questions covers:
Definitions for solution, solute, solvent, insoluble, soluble.
The technique of filtration
The technique of crystallisation

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Simple Distillation
This PowerPoint presentation with worked examples and student questions covers:
• Changes of state
• The technique of simple distillation
• Concentration of solute increasing in distillation
• Jobs related to chemistry
• Key word test Insoluble, Soluble, Solvent, Solute, Solution, Distillation, Filtration, and Crystallisation

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

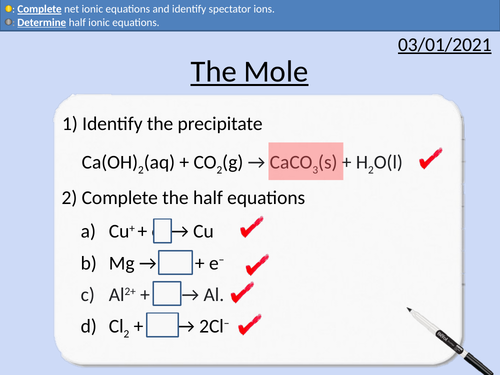
GCSE Chemistry: The Mole
This PowerPoint presentation with worked examples and student questions covers:
• Using Standard Form
• Avogadro’s constant
• Relative Atomic Mass, Relative Formula Mass and Molar Mass
• Rearranging Equations
• Calculating the number of moles present

GCSE Chemistry: Bond Energies and Energy Changes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of bond energies
• Calculating bond energies per mole
• Calculating change in bond energies in reactions
• Determining if a reaction is exothermic or endothermic from the change in bond energy.

GCSE Chemistry: Exothermic and Endothermic Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition for exothermic and endothermic
• Examples of exothermic and endothermic reactions
• Practical procedure for NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
• Determining if experimental evidence show a exothermic or endothermic reaction

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases
Bundle

GCSE OCR Chemistry C4.1 Predicting and identifying reactions and products
C4.1 Predicting and identifying reactions and products
All resources for P4.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Group 1 - The Alkali Metals
Group 7 - The Halogens
Halogen Displacement Reactions
Group 0 - The Noble Gases
The Transition Metals
Reactivity of Elements

OCR AS Chemistry: Properties of Alkenes
OCR AS Chemistry: 13.1 Properties of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.

OCR AS Chemistry: Electrophilic Addition in Alkenes
OCR AS Chemistry: 13.4 Electrophilic Addition in Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule

OCR AS Chemistry: Properties of Alcohols
OCR AS Chemistry: 14,1 Properties of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces

OCR AS Chemistry: The Chemistry of Haloalkanes
OCR AS Chemistry: The Chemistry of Haloalkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming Haloalkanes
Classifying Haloalkanes (primary, secondary, tertiary)
Electronegativity
Reaction mechanism for hydrolysis
Rates of reactions for hydrolysis
Reaction conditions for hydrolysis
Bundle

OCR AS level Chemistry: Organic Synthesis
OCR AS level Chemistry: Organic Synthesis is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Heating under reflux
Distillation
Re-distillation
Purifying Organic Products
Removing impure acids from organic compounds
Drying agents
Functional Groups - Alkane, Alkene, Haloalkane, Alcohols, Carboxylic Acid, Ketone, Aldehyde, Ester, Amine, Nitrile.
One-step synthetic routes with reagents and conditions
Two-step synthetic routes with reagents and conditions
Bundle

OCR AS level Chemistry: Alcohols
OCR AS level Chemistry: Alcohols is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces
Combustion of alcohols
Reflux condition for reactions
Primary alcohol to aldehydes
Primary alcohols to carboxylic acids
Secondary alcohols to ketones
Dehydration of alcohols
Substitution reactions for alcohols

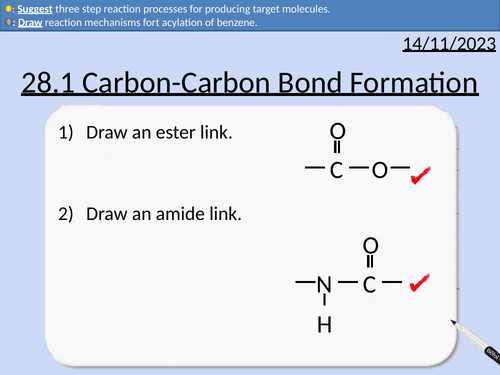
A level Chemistry: Carbon-Carbon Bond Formation
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Forming nitriles from haloalkanes
Forming nitriles from aldehydes and ketones
Forming amines from nitriles (reduction)
Forming carboxylic acids from nitriles (hydrolysis)
Friedel-Crafts alkylation of benzene
Acylation of benzene with acyl chloride

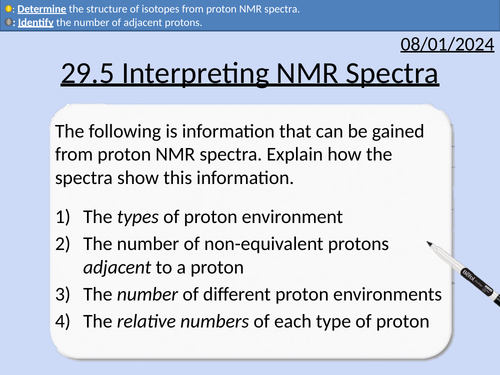
A level Chemistry: Interpreting Proton NMR Spectra
OCR A level Chemistry: 29.5 Interpreting Proton NMR Spectra
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Predicting proton NMR spectra for molecules
Identifying the number of different proton environments
Identifying the types of proton environment and chemical shifts
Integration traces (area of peaks) and relative number of protons
The spin-spin splitting pattern (n + 1)

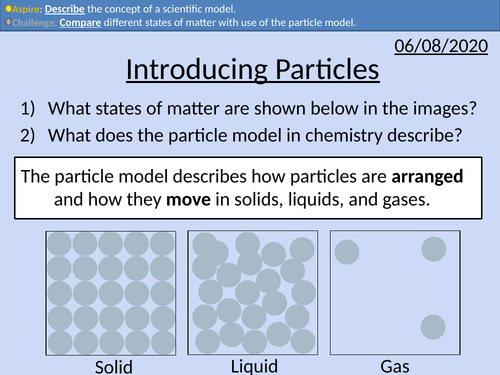
GCSE Chemistry: Introducing Particles
This PowerPoint presentation with worked examples and student questions covers:
• Solids, liquids, and gases
• Scientific models as a concept
Bundle

GCSE OCR Chemistry: P1.2 Atomic Structure
All resources for P1.2 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Atomic Structure
Isotopes and Ions
Developing the Atomic Model