497Uploads
167k+Views
71k+Downloads
Chemistry

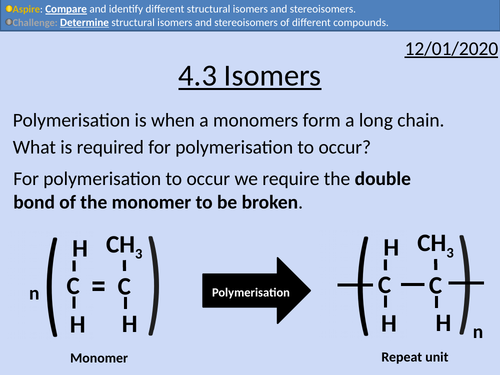
OCR Applied Science: 4.3 Isomers
This PowerPoint presentation with worked examples and student activities covers: Topic 4.3 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
• Stating definitions and comparing structural isomers and stereoisomers.
• Condensed structural formula
• Lines of symmetry for structural isomers
• Cis- and Trans isomers
• Optical isomers as non-superimposable mirror images.
• Wedge and Dash Notation
• Identifying chiral centres (asymmetric carbons)
• Le Bel-van’t Hoff rule
• Determining the maximum number of isomers.
Bundle

OCR AS level Chemistry: Alkenes
OCR AS level Chemistry: Alkenes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.
E/Z isomerism
Conditions for trans- and cis- isomerism
Cahn-Ingold-Prelog rules and priority ordering
Alkene addition reactions:
Hydrogen with a nickel catalyst
Halogens
Hydrogen halide
Steam with an acid catalyst
Test for unsaturated alkenes.
Bond enthalpy for sigma and pi bonds.
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule
Monomers and repeat units
Addition Polymerisation for:
Polyethene
Polypropene
Polylactate
Polystyrene
Polyvinyl Chloride (PVC)
Environmental Concerns from polymers including:
Combustion of polymers
recycling PVC
biogradeable bioplastics
photodegradable polymers
feedstock recycling
Bundle

OCR AS level Chemistry: Haloalkanes
OCR AS level Chemistry: Haloalkanes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Naming Haloalkanes
Classifying Haloalkanes (primary, secondary, tertiary)
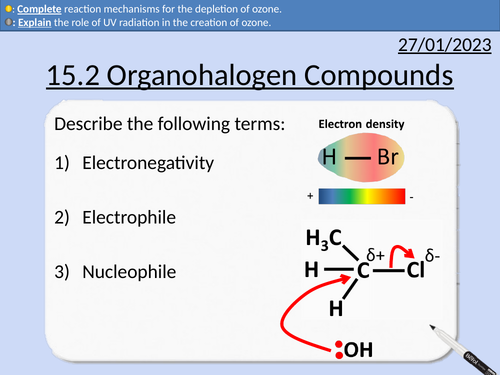
Electronegativity
Reaction mechanism for hydrolysis
Rates of reactions for hydrolysis
Reaction conditions for hydrolysis
Definitions for CFC (Chlorofluorocarbons) and HCFC (Hydachlorofluorocarbons)
Creation of ozone
Depletion of ozone with CFCs
Reaction steps including initiations and propagation

OCR AS Chemistry: Organohalogen Compounds
OCR AS Chemistry: 15.2 Organohalogen Compounds and the Environment
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definitions for CFC (Chlorofluorocarbons) and HCFC (Hydachlorofluorocarbons)
Creation of ozone
Depletion of ozone with CFCs
Reaction steps including initiations and propagation

A Level Chemistry: The Chemistry of Phenol
OCR A level Chemistry: 25.3 The Chemistry of Phenol
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming phenols
Distinguishing between phenols and alcohols
Distinguishing between phenols and alkenes
Distinguishing between phenols and carboxylic acids
Phenol as a weak acid
Electrophilic reactions with phenols
Comparing and explaining the reactivity of phenols and benzene

A level Chemistry: Carbon-13 NMR Spectroscopy
OCR A level Chemistry: 29.3 Carbon-13 NMR Spectroscopyy
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Identifying different carbon environments
The types of carbon environment
The amount of chemical shift ẟ / ppm

A level Chemistry: Nuclear Magnetic Resonance (NMR) Spectroscopy
OCR A level Chemistry: 29.2 Nuclear Magnetic Resonance (NMR) Spectroscopy
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Nuclear Spin
Resonance
Tetramethylsilane (TMS)
Chemical Shift ẟ

GCSE Chemistry: Atomic Structure
This PowerPoint presentation with worked examples and student questions covers:
• Scientific models as a concept
• Structure of the atom
• Relative mass and charge of subatomic particles
• Bond length of atoms and molecules

GCSE Chemistry: Development of the Atomic Model
This PowerPoint presentation with worked examples and student questions covers:
• Dalton, Thomson, Rutherford and Bohr’s models
• Comparing different scientific models of the atom

OCR Applied Science: 1.3 Ionic and Covalent Bonding
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.3 of Science Fundementals of the OCR Applied Science Spec.
Elements react together to form compounds by i.e.
ionic bonding
covalent bonding

OCR Applied Science: 4.2 Polymers and Carbon Compounds
This PowerPoint presentation with worked examples and student activities covers:
Topic 4.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Determining the empirical formula for compounds
Draw monomers and repeat units using structural and skeletal formula of the following polymers:
Polyethene
Polypropene
Polylactate
Polystyrene
Polyvinyl chloride (PVC)

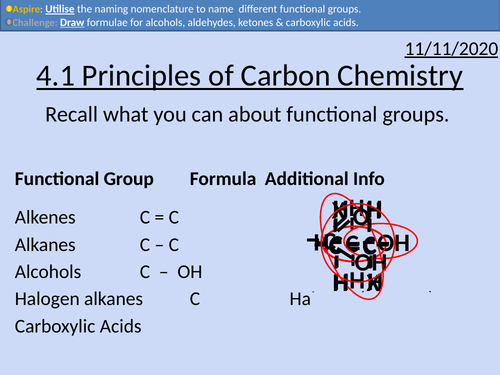
OCR Applied Science: 4.1 Principles of Carbon Chemistry
This PowerPoint presentation with worked examples and student activities covers:
Topic 4.1 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
• Alkanes as saturated hydrocarbons containing single C-C and C-H bonds
• Alkenes as unsaturated hydrocarbons containing a C=C double bond
• Alkynes as unsaturated hydrocarbons containing a C ≡ C triple bond
• Name and draw structural and skeletal formulae of the first four members of alkanes, alkenes and alkynes
• Aldehydes and ketones as organic compounds containing the C=O group
• Name and draw the structural formulae of the first four aldehydes and the first two ketones
• Alcohols as organic compounds containing the OH group
• Name and draw structural and skeletal formulae of the first four alcohols
• Conversion of alcohols to form aldehydes and ketones is classified as an oxidation reaction
• Name and draw structural and skeletal formulae of the first four carboxylic acids
• Reaction of carboxylic acids with an alkali, to include full equations using structural formulae
• Name and draw structural and skeletal formulae of the four C4H8O2 esters
• How an ester can be made from a carboxylic acid and an alcohol

GCSE Chemistry: Simple Molecules
This PowerPoint presentation with worked examples and student questions covers:
• Dot and cross diagrams of simple molecules
• Simple molecules form covalent bonds
• The group number on the periodic table informs us how many electrons are in the outer shell.
• Groups on the periodic table

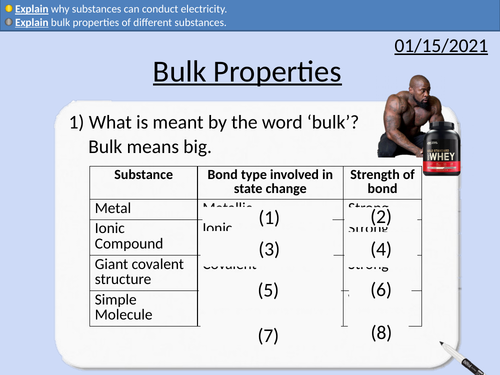
GCSE Chemistry: Bulk Properties
This PowerPoint presentation with worked examples and student questions covers:
• Jobs in Material Science
• Bulk properties of metals - malleable and conductors of electricity
• Bulk properties of ionic and covalent structures - brittle
• Explain why substances conducting electricity depends upon the state of matter

OCR Applied Science: 21.1 Regulatory Bodies
This PowerPoint presentation with worked examples and student activities covers: Topic 1.1 and 1.2 of Module 21: Product Testing Techniques.
Understand the influence of regulatory bodies on development of consumer products.
1.1 The relevant governing bodies that oversee product safety for
manufacturers and consumers of products.
1.2 How governing bodies influence how quality control is applied.
Bundle

OCR Applied Science: 21.2 Product Testing of Consumer Products
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
2.3 Effectiveness of test i.e.:
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use (e.g. toxicity, possible mutagenic and
teratogenic effects, microbiological safety)

GCSE Chemistry: Nanoparticles
This PowerPoint presentation with worked examples and student questions covers:
• Relative size of nanoparticles
• Convert nanometres using standard form
• Uses and dangers of nanoparticles

GCSE Chemistry: Covalent Structures
This PowerPoint presentation with worked examples and student questions covers:
• Definition of giant covalent structures
• An empirical formula shows the simplest whole-number ratio of the atoms of each compound.
• Melting and boiling point of simple molecules
• Compare physical properties of simple molecules and giant covalent lattices.

GCSE Chemistry: Conservation of Mass
This PowerPoint presentation with worked examples and student questions covers:
• State the number of atoms from a chemical formula.
• Relative Atomic masses and relative formula mass
• Practical activity of non-closed chemical reactions.

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals