497Uploads
167k+Views
71k+Downloads
All resources

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Simple Distillation
This PowerPoint presentation with worked examples and student questions covers:
• Changes of state
• The technique of simple distillation
• Concentration of solute increasing in distillation
• Jobs related to chemistry
• Key word test Insoluble, Soluble, Solvent, Solute, Solution, Distillation, Filtration, and Crystallisation

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

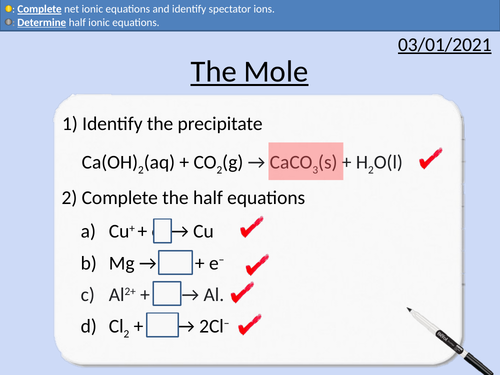
GCSE Chemistry: The Mole
This PowerPoint presentation with worked examples and student questions covers:
• Using Standard Form
• Avogadro’s constant
• Relative Atomic Mass, Relative Formula Mass and Molar Mass
• Rearranging Equations
• Calculating the number of moles present

GCSE Chemistry: Bond Energies and Energy Changes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of bond energies
• Calculating bond energies per mole
• Calculating change in bond energies in reactions
• Determining if a reaction is exothermic or endothermic from the change in bond energy.

GCSE Chemistry: Exothermic and Endothermic Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition for exothermic and endothermic
• Examples of exothermic and endothermic reactions
• Practical procedure for NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
• Determining if experimental evidence show a exothermic or endothermic reaction

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases
Bundle

GCSE OCR Chemistry C4.1 Predicting and identifying reactions and products
C4.1 Predicting and identifying reactions and products
All resources for P4.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Group 1 - The Alkali Metals
Group 7 - The Halogens
Halogen Displacement Reactions
Group 0 - The Noble Gases
The Transition Metals
Reactivity of Elements

OCR AS Physics: Electrical Energy & Power
OCR AS Physics: Electrical Energy & Power is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Derive three equations for electrical power
Applying electrical power equations
Create a circuit diagram to calculate power
Base units for V A and W.

OCR AS Physics: Reflection & Refraction
OCR AS Physics A: Reflection & Refraction is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

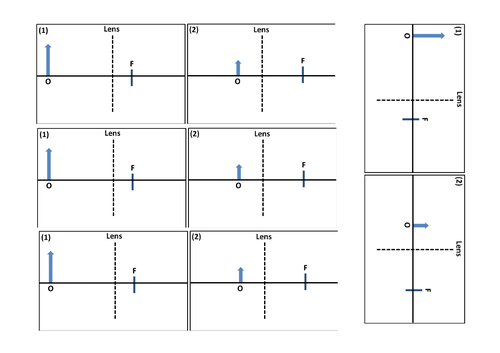
GCSE Physics: Lenses
This presentation covers OCR Gateway Physics 9-1 P5.3.2
Includes student activities and full worked answers.
Convex and Concaves lenses
Eyes and corrective lenses
Refraction and wavelength
Focal points for lenses
Determining the type of images produced through a lens

GCSE Physics: Nuclear Equations
This presentation covers OCR Gateway Physics 9-1 P6.1.3 Nuclear Equations
All presentations come with student activities and worked solutions.
Nuclear Notation - mass number and atomic number
Nuclear equations for emissions of:
Alpha radiation
Beta radiation
Gamma radiation

GCSE Physics: Electromagnetic Waves
These presentations cover the OCR Gateway Physics 9-1 P5.2.1 Electromagnetic Waves. Includes student activities and full worked answers.
Order of the electromagnetic spectrum
Wavelength and frequency relationship
Application of wave speed equation
Rearranging equation
Producing and detecting radio waves

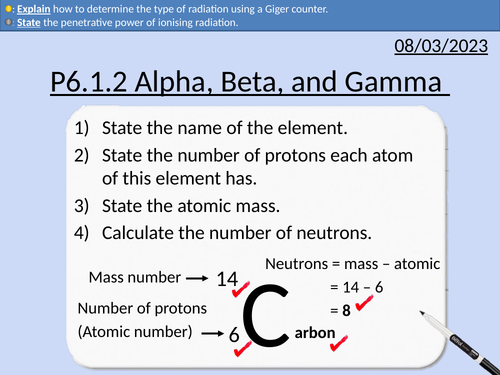
GCSE Physics: Alpha, Beta, Gamma Radiation
This presentation covers OCR Gateway Physics 9-1 P6.1.2 Alpha, Beta, Gamma Radiation.
All presentations come with student activities and worked solutions.
Types of radiation - alpha, beta, gamma, and neutrons
Ions and Atoms
Ionising Radiation
Geiger counter
Range and penetrative power
Precautions using radiation sources
Exam question with solution
Bundle

GCSE OCR Physics: P7.1 Energy and Forces
All resources for P7.1 Work Done GCSE OCR Physics Gateway 9-1. Triple and combined (Higher and Foundation) is covered in this material.
Energy Stores and Energy Transfers
Work Done and Kinetic Energy
Work Done, Kinetic Energy and Thermal Energy
Work Done and Spring Energy
Gravitational Energy
Kinetic Energy
Kinetic and Gravitational Energy
Gravitational and Spring Energy
Gravitational Force and Energy

OCR AS Chemistry: Electrophilic Addition in Alkenes
OCR AS Chemistry: 13.4 Electrophilic Addition in Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule

OCR AS Chemistry: Properties of Alcohols
OCR AS Chemistry: 14,1 Properties of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces

OCR AS Chemistry: The Chemistry of Haloalkanes
OCR AS Chemistry: The Chemistry of Haloalkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming Haloalkanes
Classifying Haloalkanes (primary, secondary, tertiary)
Electronegativity
Reaction mechanism for hydrolysis
Rates of reactions for hydrolysis
Reaction conditions for hydrolysis
Bundle

OCR AS level Chemistry: Organic Synthesis
OCR AS level Chemistry: Organic Synthesis is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Heating under reflux
Distillation
Re-distillation
Purifying Organic Products
Removing impure acids from organic compounds
Drying agents
Functional Groups - Alkane, Alkene, Haloalkane, Alcohols, Carboxylic Acid, Ketone, Aldehyde, Ester, Amine, Nitrile.
One-step synthetic routes with reagents and conditions
Two-step synthetic routes with reagents and conditions
Bundle

OCR AS level Chemistry: Alcohols
OCR AS level Chemistry: Alcohols is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces
Combustion of alcohols
Reflux condition for reactions
Primary alcohol to aldehydes
Primary alcohols to carboxylic acids
Secondary alcohols to ketones
Dehydration of alcohols
Substitution reactions for alcohols