497Uploads
167k+Views
71k+Downloads
All resources

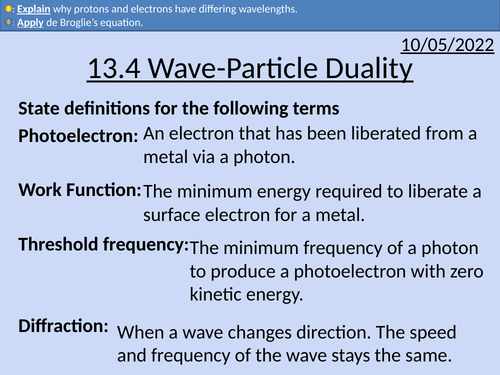
OCR AS level Physics: Wave-Particle Duality
OCR AS level Physics: Wave-Particle Duality is a part of the Module 4: Electrons, Waves, and Photons.
Full lesson PowerPoint with worked examples and homework with complete worked answers.
deBroglie wavelength equation
Diffraction of electrons and protons
Comparing wavelengths of particles with different masses
Kinetic energy and wavelength
Bundle

GCSE OCR Chemistry: P1.2 Atomic Structure
All resources for P1.2 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

GCSE Chemistry: Empirical Formula
This PowerPoint presentation with worked examples and student questions covers:
• Calculate empirical formula and by finding the simplest whole-number ratio
• Calculate relative formula mass from balanced equations.

OCR Applied Science: 1.2 The Periodic Table
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.2 of Science Fundementals of the OCR Applied Science Spec.
Elements are based on atomic structure and can be classified by the Periodic Table i.e.:
organisation of elements within the table
groups
periods
atomic number
atomic mass atomic radius

OCR Applied Science: 2.2 Reactions
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.2 of Module 1: Science Fundementals of the OCR Applied Science Spec.
Oxidation and reduction (redox) reactions
Addition reactions of alkenes to include full balanced symbol equations
Substitution reactions of alkanes and haloalkanes to include full balanced
equations
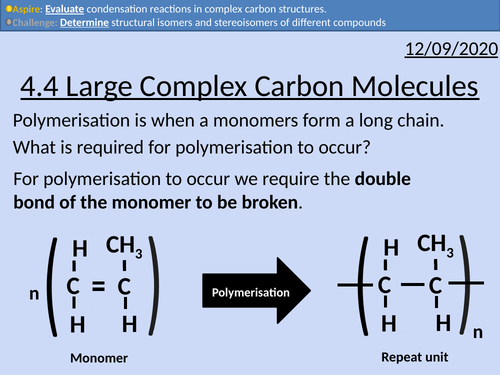
Addition polymerisation to include identification of monomers and repeating units
Condensation polymerisation to include identification of monomers and repeating units
Definition of a radical
The role played by UV light in producing chlorine radicals from CFCs in the
depletion of the ozone layer
Equations to show how chlorine radicals can destroy many ozone molecules
Displacement reactions to include full balanced equations for metals and halogens.

OCR Applied Science: 6.2 Physico-chemical Properties of Materials
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Structure of metals, giant covalent, and simple molecular structures.
Properties of metals, giant covalent, and simple molecular structures.
Forces and bonds of metals, giant covalent, and simple molecular structures.
Phase diagrams – interpreting and calculating changes.
Sublimation and phase diagrams.

OCR Applied Science: 6.3 Electrical Properties
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.3 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Current as flow of charge in a conductor.
Use the equation: I = ΔQ ÷ Δt
Ohm’s law illustrates the relationship of V ∝ I
Use the equation: potential difference (V) = current (A) × resistance
Use the equations for adding resistors in series and parallel
Compare electromotive force and potential difference
Use the equation: charge © = current (A) × time (s)
Use and recognise the equation for mean drift velocity
Use the equation: energy transferred (work done) (J) = charge © × potential difference (V)
Use the equation: energy transferred (J, kWh) = power (W, kW) × time (s, h)
Use the equation: power (W) = energy (J) ÷ time (s)

GCSE Chemistry: Purification and Checking Purity
This PowerPoint presentation with worked examples and student questions covers:
• Choosing the correct separation technique
• Comparisons of mobile and stationary phases for chromatography
• Rf Values
• Analysing chromatographs in gas chromatography

OCR Applied Science: 4.4 Large Complex Carbon Molecules
This PowerPoint presentation with worked examples and student activities covers: Topic 4.4 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Complex carbohydrates (starch, glycogen, cellulose)
• Carbohydrates found as monosaccharides, disaccharides, or polysaccharides (monomers, dimers or polymers)
• Monomers held together by glycosidic bonds to form dimers and polymers, via condensation reactions
• Monosaccharides include glucose, fructose and galactose
• Disaccharides include maltose, sucrose and lactose
• Polysaccharides include starch, glycogen and cellulose
• Cellulose is found in plant cell walls where it provides strength/support and pliability
• Starch and glycogen are energy sources
Proteins and peptides from amino acids
• Dipeptides are formed from two amino acids joined by a peptide bond, via a condensation reaction
• Polypeptides are chains of amino acids joined by peptide bonds
• Proteins/polypeptides have physiological or functional roles, including enzymes, carrier proteins in the plasma membrane, and structural roles, including collagen and elastin fibres in connective tissue
Lipids from fatty acids, glycerol and phosphorus compounds
• Monoglycerides, diglycerides and triglycerides are esters of fatty acids and glycerol
• An ester bond forms between each fatty acid and the glycerol, via condensation reactions
• Phospholipids contain glycerol plus two fatty acids and a phosphate group
• Lipids act as an energy source within cells, as an insulation layer around animal organs, in the myelin sheath (found around some nerve fibres/axons) to increase speed of nerve transmission
• Phospholipids form a bilayer in the plasma membrane
Protein synthesis (transcription, translation) RNA, messenger, ribosomal and transfer
• The nucleic acids, DNA and RNA, are polymers of nucleotides
• Peptide bonds form between amino acids to create polypeptide chains/proteins
• Recall a simple description of protein synthesis

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).

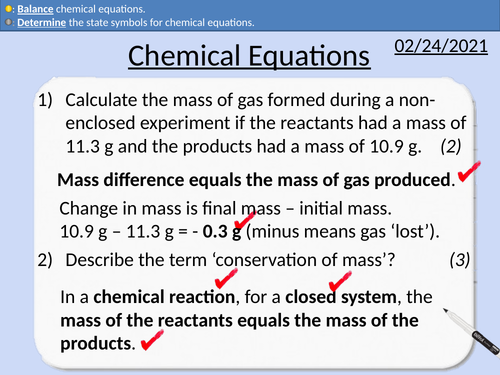
GCSE Chemistry: Chemical Equations
This PowerPoint presentation with worked examples and student questions covers:
• Pathways into medical chemistry
• State the number of atoms from a chemical formula.
• Properties of metals and non-metals
• Determine state symbols for chemical equations
• Balancing chemical equations

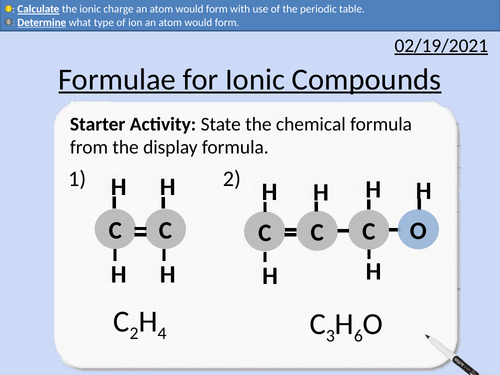
GCSE Chemistry: Formulae for Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• State the number of electrons in each energy level.
• Determine what type of ion an atom would form.
• Calculate the ionic charge an atom would form with use of the periodic table.
• Groups number, outer shell electrons, dot and cross diagrams
Bundle

GCSE OCR Chemistry C2 Elements, Compounds, and Mixtures
Resources for P2 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Relative Formula Mass
Empirical Formula
Pure and Impure Substances
Filtration and Crystallisation
Simple Distillation
Paper Chromatography
Purification and Checking Purity
Metals and Non-metals
Electronic Structures
Forming Ions
Ionic Compounds
Simple Molecules
Giant Covalent Structures
Polymer Molecules
Structure of Metals
Carbon
Changing State
Bulk Properties
Nanoparticles

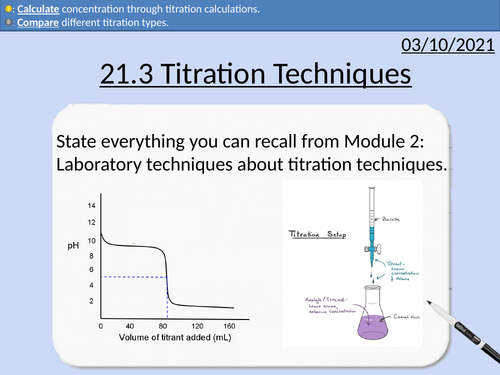
OCR Applied Science: 21.3 Titration Techniques
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
3.1 Titration techniques on consumer products
• Acid-base titration (e.g. limescale removers, eco-disinfectants)
• Precipitation titration (e.g. contact lens saline solution)
• Redox titration, (e.g. bleach, tooth whitener; vitamin C tablets).
• Complexometric titrations (e.g. Milk of Magnesia)
Including explanation and activities on:
Titration calculations
Moles and molar mass
Rearranging Equations
State symbols
Significant Figures
Comparing Data

GCSE Chemistry: Electrolysis of Water
This PowerPoint presentation with worked examples and student questions covers:
• Pure water being made partially of ions (hydrogen and hydroxide).
• PANIC convention for electrodes
• OILRIG convention for redox reactions
• Electron transfers at electrodes
• Half-equations for anode and cathode
• Balancing half-equations

GCSE Chemistry: Group 1 - Alkali Metals
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Alkali Metals
• Trends and anomalies in Group 1 (Density, Melting Point)
• Reactivity of Group 1 Alkali Metals
• Electron configuration of Group 1 Alkali Metals

GCSE Chemistry: Detecting Cations
This PowerPoint presentation with worked examples and student questions covers:
Flame tests for lithium, sodium, potassium, calcium, and copper.
Electron energy levels and emitting radiation.
Precipitate tests for iron(II)), iron(III), copper(II), calcium, and zinc.

GCSE Chemistry: Detecting Gases
This PowerPoint presentation with worked examples and student questions covers:
Tests for Hydrogen, Oxygen, Carbon Dioxide, Chlorine.
Gifs of each gas test
Electron structure for diatomic molecules

GCSE Chemistry: Biological Polymers
This PowerPoint presentation with worked examples and student questions covers:
Proteins as polymers and amino acids as monomers
Carbohydrates and simple sugars
Comparing simple sugars (glucose, fructose, and sucrose) with complex carbohydrates (starch).
DNA as a polymer and nucleotides as monomers
Structure of nucleotides (phosphate group,
a sugar (deoxyribose), and an organic base).
Base pairing in DNA and hydrogen bonds